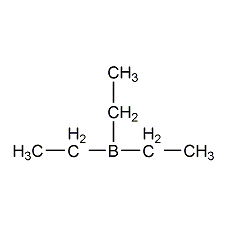
structural formula
| business number | 02cx |
|---|---|
| molecular formula | c6h15b |
| molecular weight | 97.99 |
| label |
ethylboron, triethylboron, triethylborane solution in tetrahydrofuran, triethylboron, boron triethyl, boron ethyl, (c2h5)3b, borane,triethyl-, borethyl, triethyl-boran |
numbering system
cas number:97-94-9
mdl number:mfcd00009022
einecs number:202-620-9
rtecs number:ed2100000
brn number:1731462
pubchem number:24855572
physical property data
1. properties: colorless, transparent fuming liquid.
2. density (g/ml, 25℃): 0.865
3. relative vapor density (g/ml, air=1): 5.0
4. melting point (oc): -93
5. boiling point (oc, normal pressure): 95
6. boiling point (oc, kpa): undetermined
7. refractive index: 1.380
8. flash point (oc): -35.6
9. specific rotation (o): undetermined
10. autoignition point or ignition temperature (oc): not determined
11. vapor pressure (mmhg, 21.1oc): not determined
12. saturated vapor pressure (kpa, 20oc ): undetermined
13. heat of combustion (kj/mol): undetermined
14. critical temperature (oc): undetermined
15. critical pressure (kpa): undetermined
16. log value of oil-water (octanol/water) distribution coefficient: undetermined
17. explosion upper limit (%, v/v): undetermined
18. lower explosion limit (%, v/v): undetermined
19. solubility: insoluble in water, soluble in ethanol and ether.
toxicological data
1. acute toxicity:
rat oral ld50: 235mg/kg;
rat inhalation lc50: 700ppm/4h;
rat peritoneal cavity ld50: 22700μg/kg;
ecological data
this substance is harmful to the environment, and special attention should be paid to the pollution of water bodies.
molecular structure data
1. molar refractive index: 33.27
2. molar volume (cm3/mol): 149.5
3. isotonic specific volume (90.2k ): 307.7
4. surface tension (dyne/cm): 17.9
5. polarizability (10-24cm3): 13.19
compute chemical data
1. hydrophobic ginsengreference value for numerical calculation (xlogp): none
2. number of hydrogen bond donors: 0
3. number of hydrogen bond acceptors: 0
4. number of rotatable chemical bonds: 3
5. number of tautomers: none
6. topological molecule polar surface area 0
7. number of heavy atoms :7
8. surface charge: 0
9. complexity: 25.7
10. number of isotope atoms: 0
11 .determine the number of atomic stereocenters: 0
12. uncertain number of atomic stereocenters: 0
13. determine the number of chemical bond stereocenters: 0
14. number of uncertain chemical bond stereocenters: 0
15. number of covalent bond units: 1
properties and stability
it can spontaneously ignite in the air and burn to produce a characteristic green flame. triethylboron readily undergoes auto-oxidation through free radical reactions in the presence of oxygen.
storage method
store in a cool, dry, well-ventilated warehouse. keep away from fire and heat sources. protect from direct sunlight. the storage temperature should not exceed 30℃. relative humidity remains below 75%. keep container sealed and strictly prohibited from contact with air. they should be stored separately from oxidants, food chemicals, etc., and avoid mixed storage. use explosion-proof lighting and ventilation facilities. it is prohibited to use mechanical equipment and tools that are prone to sparks. the storage area should be equipped with emergency release equipment and suitable containment materials.
synthesis method
1. in a 1-liter three-necked flask equipped with a mercury-sealed stirrer, reflux cooler, dropping funnel and air guide, put 72 grams (3.0 grams atoms) of magnesium chips and 250 ml of anhydrous water. butyl ether. under nitrogen protection, slowly add 500 ml of anhydrous butyl ether solution containing 327 g (3.0 mol) of ethane to prepare grignard reagent. the reaction started very violently, so the flask must be immersed in a cold water bath to cool, and then 400 ml of butyl ether solution containing 61 g (0.9 mol) boron trifluoride was added dropwise within 4 hours. afterwards, heat at 70°c for 2 hours. (after the reaction is completed, replace the condenser with a ground glass stopper, and the unsealed stirrer with a short fractionator. . such changes in the apparatus should be carried out quickly with a rapid nitrogen flow to avoid exposure of the mixture to air). directly distill the reaction mixture and collect the fractions at 94—97°c to obtain 65 grams (74%) of crude triethylboron.
during purification, the crude product can be reacted with slightly passed ammonia to form a complex. after this complex is evaporated under high vacuum, it reacts with a slight excess of dry hydrogen to free triethylboron. redistilled and collected in cold hydrazine at -40°c.
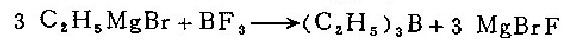
purpose
1. used in organic synthesis, mixed with triethylaluminum and used as a two-component igniter in rocket propulsion systems.
2. selective aldol condensation reaction. a-alkylation of ketones.
3. the greatest use of triethylboron is as a free radical initiator. it can not only initiate reactions at low temperatures (–78 oc), but can also be used for free radical reactions. brings good stereoselectivity. at the same time, triethylboron can also be used in non-radical reactions, such as promoting the three-component michael-aldol condensation allylation reaction of palladium-catalyzed methylene compounds, ammonia and inactive allyl alcohol.
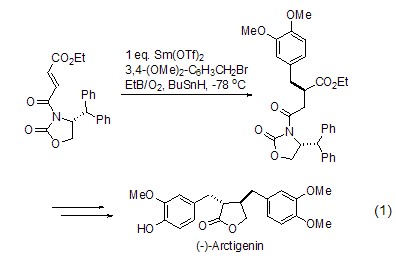
as a free radical initiator, triethylboron can be used for the enantioselective synthesis of the natural product butyrolactone (formula 1)[1]. the reaction begins with lewis-induced radical addition, leading to the final product.
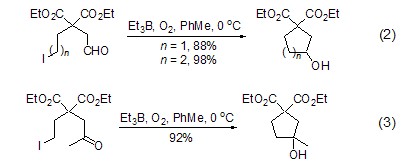
triethylboron combined with oxygen it can also realize the addition reaction of intramolecular alkane radicals to aldehydes and ketones without the participation of tin. for example, ω-iodoaldehyde can effectively undergo intramolecular formation under the induction of 10 times triethylboron. ring reaction (formula 2) [2], ω-iodoketone can also obtain the ring-forming product in high yield under 20 times of triethylboron induction (formula 3 ).
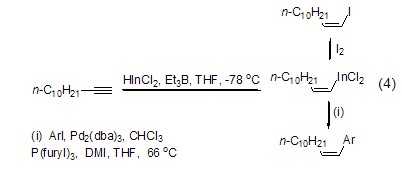
triethylboron can also be used in the hydroindiumation reaction of alkynes and alkenes (formula 4)[3]. the alkenyl indium produced by the reaction can undergo a one-step cross-coupling reaction with halogenated aromatic hydrocarbons or other electrophiles.
triethylboron can also be substituted metal reagents realize the tandem michael-aldol reaction and synthesize α-alkyl-β-hydroxyketone derivatives (formula 5) with high yields[4].

reactive submersible ions catalyzed by palladium reagents in the allylation reaction between methyl compounds and inactive allyl alcohol, triethylboron can play a very good role in promoting the c-o bond of allyl alcohol (formula 6)[5].

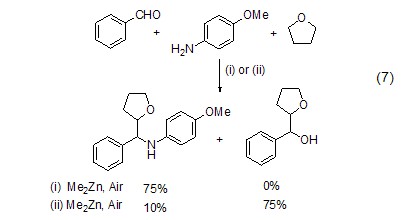
chemical selection in free radical control in the three-component addition reaction of thf free radicals with aldehydes and imines, triethylboron can selectively obtain the addition product of thf to aldehydes (formula 7)[6].
extended-reading:http://www.ksgj2020.com/archives/1769extended-reading:https://www.bdmaee.net/dimethylaminoethoxyethanol-cas-1704-62-7-n-dimethylethylaminoglycol/extended-reading:http://www.ksgj2020.com/archives/1023extended-reading:https://www.bdmaee.net/wp-content/uploads/2016/06/addocat-108.pdfextended-reading:https://www.cyclohexylamine.net/aeea/extended-reading:http://www.ksgj2020.com/archives/45090extended-reading:http://www.ksgj2020.com/archives/category/products/page/15extended-reading:http://www.ksgj2020.com/archives/40271extended-reading:https://www.morpholine.org/high-quality-cas-108-01-0-nn-dimethyl-ethanolamine-2-dimethylamineethanol-dmea-dimethylethanolamine/extended-reading:https://www.morpholine.org/potassium-acetate-glycol-solution-polycat-46/

